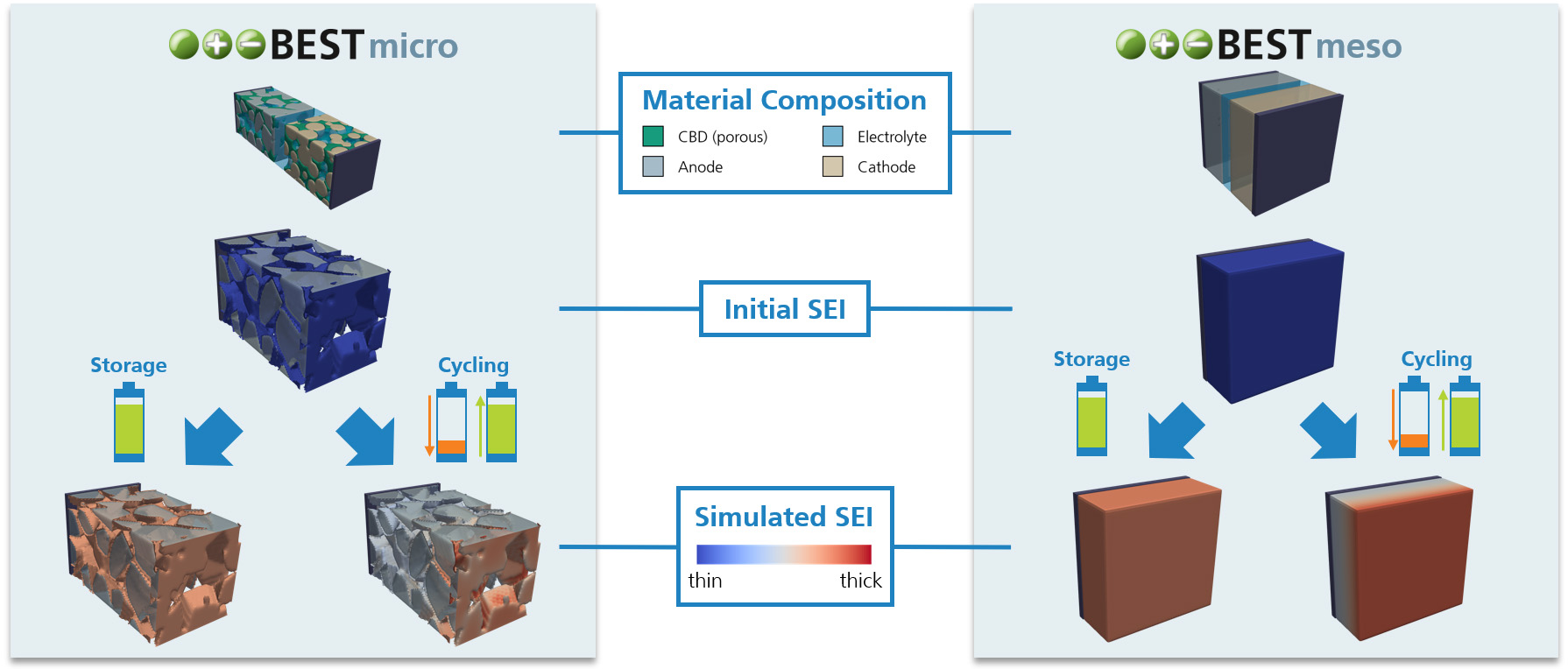
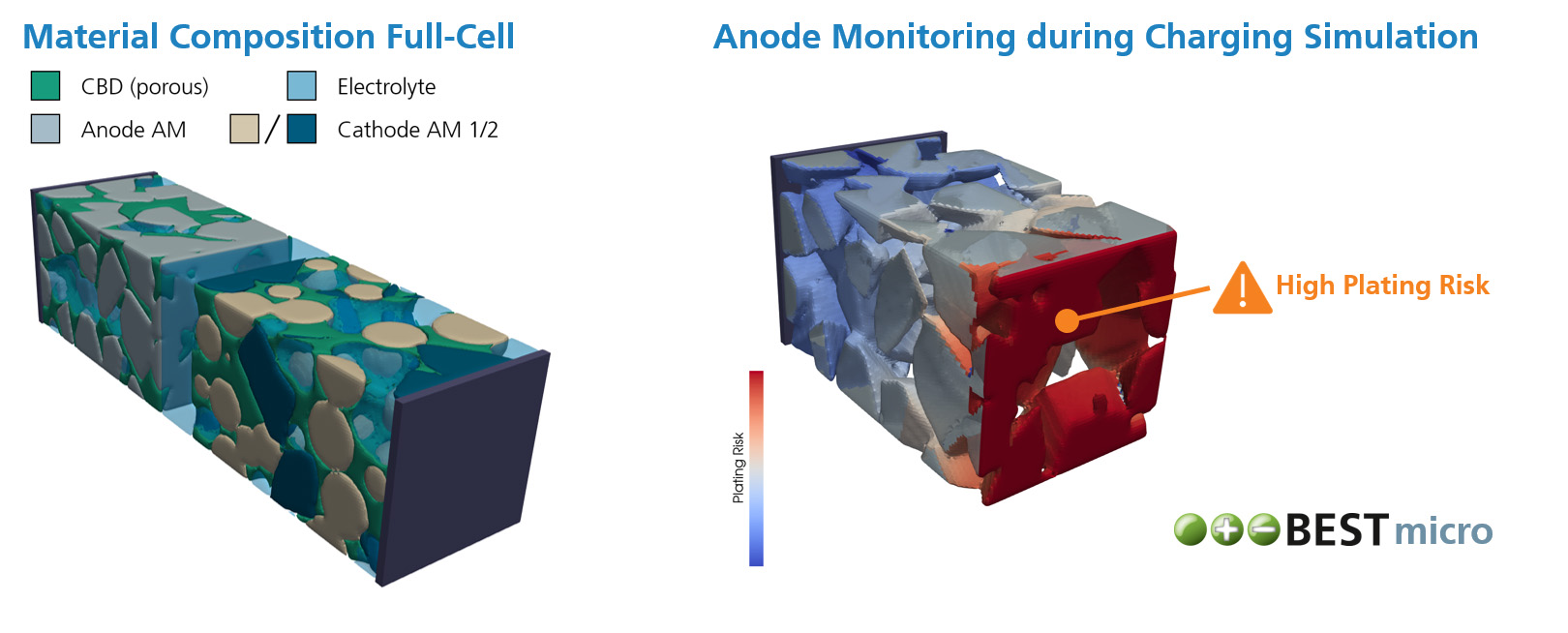
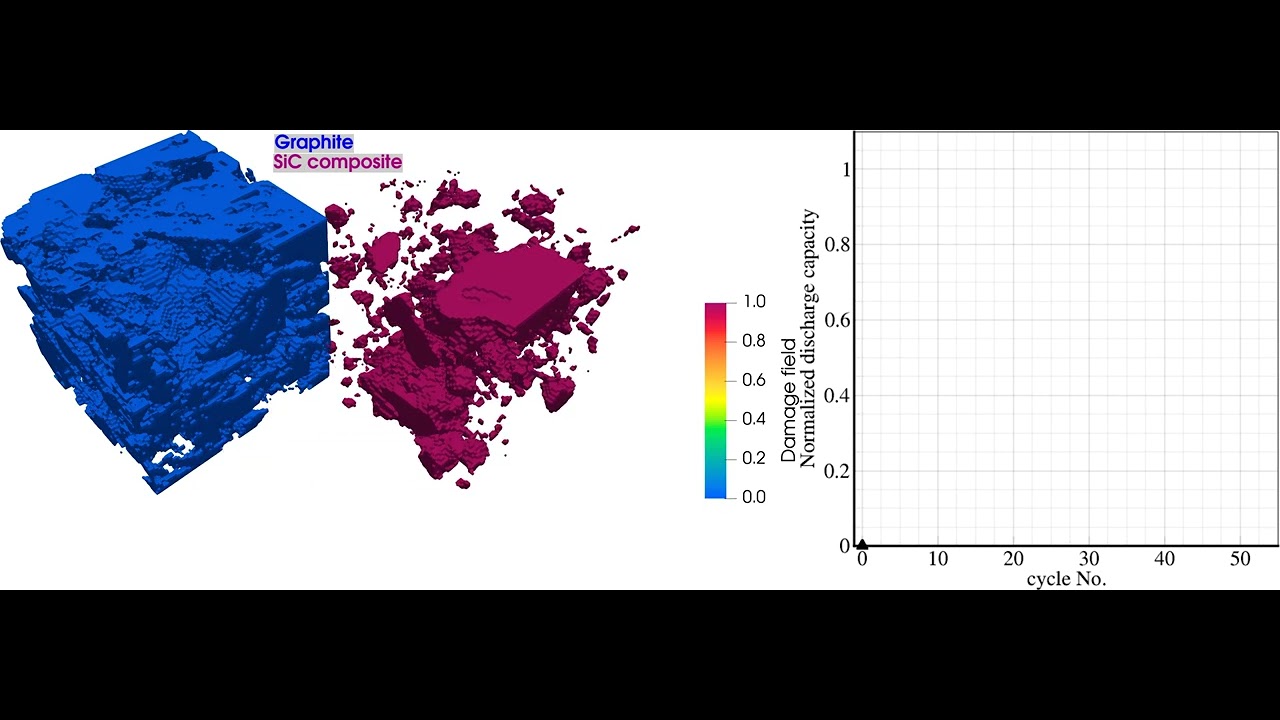
During operation and even storage, Li-ion batteries are exposed to a variety of degradation processes which cause cell aging. As a result, the cell often suffers from capacity and power fade, as well as increased heating during operation and higher risks of thermal runaway. Within different research projects we have used our simulation software BESTto consider three of the major degradation processes inside Li-ion batteries:
- Simulation of the Solid Electrolyte Interphase (SEI) Layer
- Detection of Lithium Plating Through Simulation
- Simulation of Mechanical Degradation
The following sections go into more detail regarding these topics. Click on the headings to go directly to the relevant paragraph.