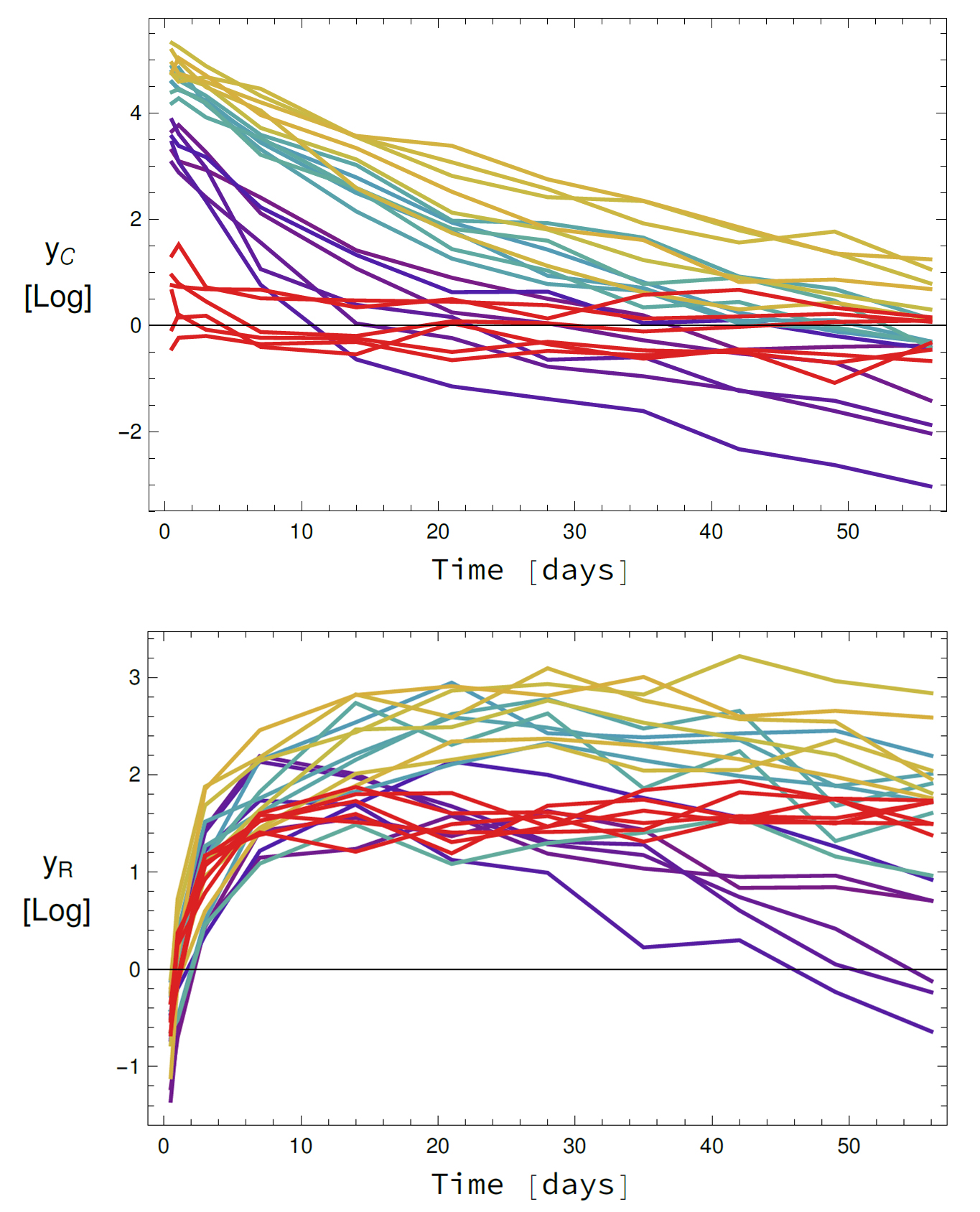
The development of new active ingredients for pharmaceutical purposes is now supported by mathematical learning methods. This capability relates to an important question: What is the proper dosage? The new remedy should work, but not harm the patient. In cooperation with our partner institute, FCC in Gothenburg, we develop and use mathematical simulation and learning methods to find an answer to the question being tested in clinical trials: What is the distribution of active ingredients throughout the body and what effects occur at the targeted sites of action?
In trial testing, as many patients as possible are given different doses of the new drug and the development of the disease in each individual patient is monitored over a long period of time. Monitoring, in this case, means: evaluation of blood samples and body functions. Unfortunately, not all processes in the body can be measured directly.